When your heart muscle weakens or stiffens, it doesn’t just affect your stamina-it can change your life. Cardiomyopathy isn’t one disease. It’s a group of conditions that attack the heart muscle directly, making it harder to pump blood. The three main types-dilated, hypertrophic, and restrictive-each behave differently, show up differently on tests, and need totally different treatments. Most people don’t know the difference until they’re diagnosed. And by then, understanding which type you have can mean the difference between managing symptoms and facing life-threatening complications.
Dilated Cardiomyopathy: The Enlarged, Weakened Heart
Dilated cardiomyopathy (DCM) is the most common form, making up about half of all cases. The heart’s main pumping chamber-the left ventricle-gets stretched out and thin, like an old balloon that’s been overinflated. Instead of squeezing hard, it just flops around. Ejection fraction drops below 40%, meaning less than half the blood is being pushed out with each beat. Normal is 55-70%.
This isn’t just about size. The walls of the ventricle get thinner than 10 mm (normal is 11-13 mm). The chamber expands beyond 55 mm in men or 50 mm in women. Blood backs up, lungs fill with fluid, and fatigue sets in fast. People with DCM often feel winded climbing stairs or carrying groceries-even if they were fit before.
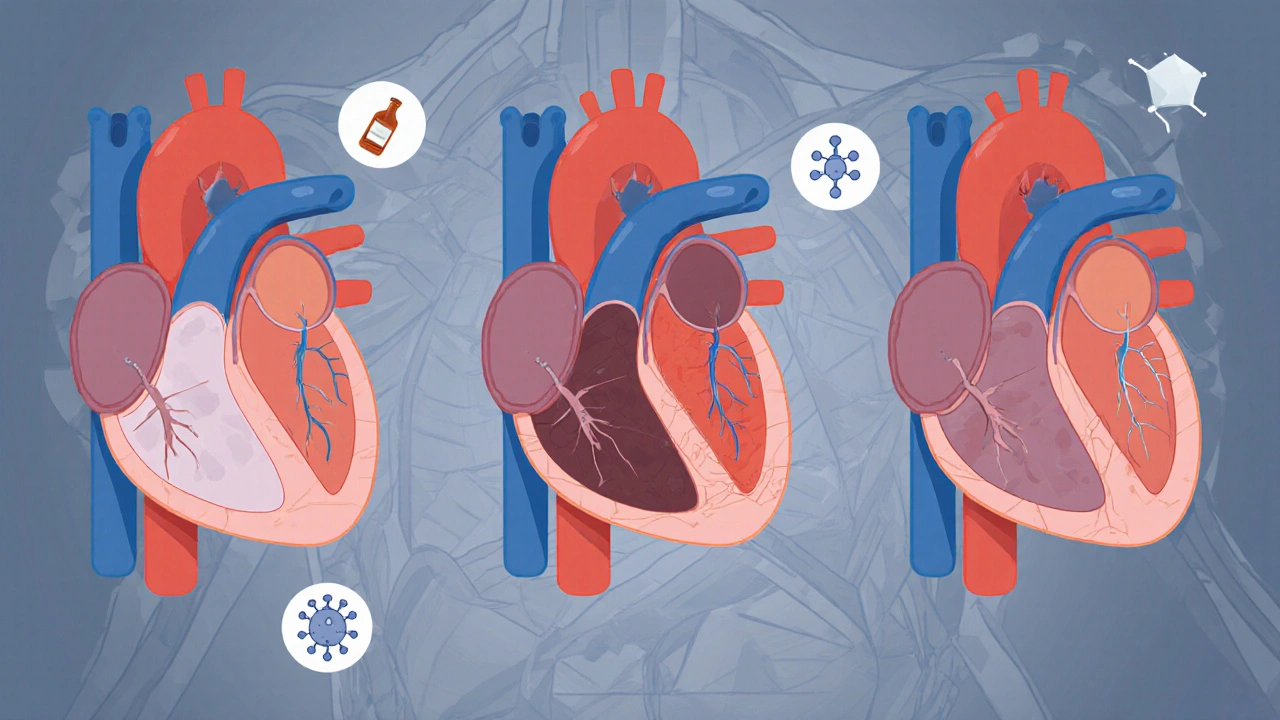
What causes it? Sometimes, it’s genetic. Mutations in genes like TTN or LMNA run in families, and about 1 in 3 cases are inherited. Other times, it’s triggered by damage: heavy alcohol use (more than 80 grams a day for years), viral infections like coxsackievirus, or chemotherapy drugs like doxorubicin. Autoimmune diseases like sarcoidosis can also sneak in and attack the heart muscle.
Diagnosis starts with an echocardiogram. If the chamber is enlarged and the pump is weak, DCM is likely. A cardiac MRI confirms it by showing scarring or inflammation. Genetic testing is recommended if there’s a family history of sudden cardiac death or early heart failure.
Treatment isn’t about fixing the shape-it’s about supporting the function. Standard therapy includes ARNIs like sacubitril/valsartan, beta-blockers, SGLT2 inhibitors (originally diabetes drugs), and aldosterone blockers. These aren’t just pills-they cut hospital stays and death risk by up to 30% over three years. In severe cases, an ICD (implantable defibrillator) prevents sudden arrest. About 10% of patients eventually need a transplant.
Hypertrophic Cardiomyopathy: The Thickened, Overworked Heart
If DCM is a stretched-out heart, hypertrophic cardiomyopathy (HCM) is a heart that’s bulking up-without reason. The muscle thickens, especially in the wall between the two lower chambers (the septum). Walls hit 15 mm or more, sometimes over 30 mm. That’s like putting a brick wall inside your heart.
Here’s the twist: the heart doesn’t always pump worse. In fact, ejection fraction is often normal or even high. The problem is stiffness. The thickened muscle can’t relax properly between beats, so the heart doesn’t fill with enough blood. That’s diastolic dysfunction. And in 70% of cases, the thickened septum blocks blood from leaving the heart-called obstructive HCM.
HCM is mostly genetic. Around 60% of cases involve mutations in sarcomere genes like MYH7 or MYBPC3. It’s passed down in families with a 50% chance of inheritance. That’s why screening relatives is critical. HCM is the number one cause of sudden cardiac death in young athletes under 35. In the U.S., it’s behind about one-third of those tragedies.
Diagnosis relies on echocardiography and cardiac MRI. If the wall is thick, and no high blood pressure or valve disease explains it, HCM is confirmed. Genetic testing finds a mutation in 60% of cases. A 17-gene panel costs between $1,200 and $2,500 in the U.S., but it’s worth it for family screening.
Treatment focuses on reducing symptoms and preventing sudden death. Beta-blockers like metoprolol help the heart relax and slow the rate. Disopyramide reduces obstruction. For severe blockage, two options exist: septal myectomy (surgical removal of excess muscle) or alcohol septal ablation (injecting alcohol to shrink the area). Both improve symptoms in 85% of patients. An ICD is recommended for those with high-risk features: family history of sudden death, unexplained fainting, or very thick walls.
Recent breakthroughs include mavacamten (Camzyos), approved in 2022. It’s the first drug designed specifically for obstructive HCM. It reduces the LVOT gradient by 80% on average. It costs $145,000 a year, but for many, it’s life-changing-replacing surgery for some.
Restrictive Cardiomyopathy: The Stiff, Unyielding Heart
Restrictive cardiomyopathy (RCM) is the rarest, making up only 5-10% of cases. But it’s one of the hardest to diagnose. The heart doesn’t get bigger or thicker-it just gets stiff. The muscle turns rigid, like cardboard instead of rubber. It can’t expand to take in blood between beats, even though the pumping action remains strong.
Key signs: normal or small ventricles, normal ejection fraction (over 50%), but severely impaired filling. Echocardiography shows a classic pattern: a spike in early filling (E wave), rapid deceleration (under 150 ms), and high pressure in the lungs. The atria get huge as they struggle to push blood into the stiff ventricles.
RCM isn’t caused by the heart itself. It’s caused by what’s invading it. Amyloidosis (60% of cases) is the biggest culprit-abnormal proteins build up in the heart tissue. Sarcoidosis (15%) causes inflammation and scarring. Hemochromatosis (10%) dumps too much iron into the muscle. Fabry disease (5%) is a genetic storage disorder.
Diagnosis requires more than an echo. Cardiac MRI shows late gadolinium enhancement in a patchy, non-coronary pattern. Blood tests check for light-chain proteins (for amyloid). Endomyocardial biopsy-the gold standard-is often needed to confirm amyloid or sarcoidosis. The problem? Many doctors don’t think of RCM. Patients wait months or years for the right diagnosis.
Treatment targets the root cause. For amyloidosis, drugs like tafamidis (costing $225,000/year) slow progression and improve walking distance by 25 meters on average. Daratumumab, a cancer drug, is now used for light-chain amyloidosis. For hemochromatosis, regular blood removal (phlebotomy) stops further damage. Steroids help with sarcoidosis.
Prognosis is grim compared to the others. Five-year survival for RCM ranges from 30% to 50%, depending on the cause. Amyloidosis has the worst outlook-unless caught early. That’s why identifying RCM quickly matters. Misdiagnosis as constrictive pericarditis (a different problem involving the heart’s outer sac) is common and dangerous-because the treatments are opposite.
How These Types Compare
| Feature | Dilated (DCM) | Hypertrophic (HCM) | Restrictive (RCM) |
|---|---|---|---|
| Main problem | Weak, enlarged pump | Thick, stiff muscle | Stiff, non-expanding chamber |
| Ventricular size | Enlarged | Normal or small | Normal or small |
| Wall thickness | Thin (<10 mm) | Thick (≥15 mm) | Normal (<12 mm) |
| Ejection fraction | <40% | Normal or high | >50% |
| Primary cause | Genetic, alcohol, virus, chemo | Genetic (sarcomere mutations) | Amyloidosis, sarcoidosis, hemochromatosis |
| Key diagnostic tool | Echocardiogram + MRI | Echocardiogram + genetic test | Echocardiogram + MRI + biopsy |
| Common treatment | ARNI, beta-blockers, SGLT2 inhibitors | Beta-blockers, septal reduction, mavacamten | Treat underlying disease (e.g., tafamidis, phlebotomy) |
| 5-year survival | 70-80% | 70-95% | 30-50% |
Why Getting the Right Type Matters
One size does not fit all. Giving a beta-blocker to someone with RCM from amyloidosis won’t fix the protein buildup. Giving tafamidis to someone with DCM won’t help their stretched muscle. Misdiagnosis leads to wrong treatment-and worse outcomes.
Doctors used to lump all heart failure together. Now, we know the type determines everything: prognosis, family risk, drug choices, and whether you need a transplant. That’s why genetic testing isn’t optional for DCM or HCM-it’s part of standard care. And why cardiac MRI is becoming routine, not just for research.
Even the newest therapies are tailored. Mavacamten works only for obstructive HCM. SGLT2 inhibitors help DCM but aren’t used for RCM. Gene therapies for HCM (like VERVE-201) are now in early trials-targeting the MYBPC3 gene to correct the root mutation.
Underdiagnosis is still a huge problem. One in 200 people have HCM, but only 10% know it. RCM is often missed because it’s rare and symptoms mimic other conditions. DCM gets diagnosed late because people blame fatigue on age or stress.

What You Can Do
If you have a family history of sudden cardiac death before age 50, unexplained heart failure, or a known genetic heart condition, get screened. An echocardiogram takes 20 minutes. It’s noninvasive. It could save your life-or your child’s.
Don’t ignore shortness of breath, especially if it’s new or getting worse. Fatigue isn’t just aging. Swelling in the legs or abdomen? That’s fluid backup. Palpitations or fainting during exercise? That’s a red flag.
And if you’ve been told you have "heart failure" without knowing the type, ask: Which kind? What caused it? Should my family be tested? The answer changes everything.
What’s the difference between dilated and hypertrophic cardiomyopathy?
Dilated cardiomyopathy means the heart chamber is stretched and weak, with thin walls and poor pumping. Hypertrophic cardiomyopathy means the heart muscle is thickened, often without enlargement, leading to stiffness and reduced filling. DCM causes low ejection fraction; HCM usually keeps it normal or high. DCM is often caused by alcohol, viruses, or genes; HCM is almost always genetic.
Can restrictive cardiomyopathy be reversed?
It depends on the cause. If it’s from hemochromatosis (iron overload), regular blood removal can stop progression and even improve function. For amyloidosis, drugs like tafamidis slow the disease but don’t reverse damage. In rare cases, early treatment can stabilize the heart. But once scarring sets in, reversal isn’t possible. The goal is to slow decline and manage symptoms.
Is cardiomyopathy hereditary?
Yes, especially dilated and hypertrophic types. About 30-40% of DCM cases and 60% of HCM cases have a genetic cause. If you’re diagnosed, your first-degree relatives-parents, siblings, children-should get screened with an echocardiogram and possibly genetic testing. Even if they feel fine, they might carry the gene.
Can you live a normal life with cardiomyopathy?
Many people do-with the right treatment. People with DCM on guideline therapy often return to work and light exercise. HCM patients may need to avoid intense sports but can live full lives with medication or surgery. RCM is harder to manage, but treating the root cause (like amyloidosis) improves quality of life. The key is early diagnosis and sticking to your treatment plan.
What’s the newest treatment for hypertrophic cardiomyopathy?
Mavacamten (brand name Camzyos) is the first drug approved specifically for obstructive HCM. It targets the heart’s overactive contractile mechanism, reducing the blockage and improving symptoms. In trials, it cut the outflow tract gradient by 80% and improved exercise capacity. It’s taken daily and requires careful monitoring due to potential heart failure risk, but for many, it’s a game-changer compared to surgery or older drugs.
How do you know if you have cardiomyopathy and not just heart failure?
Heart failure is a syndrome-symptoms like shortness of breath and swelling. Cardiomyopathy is the specific disease causing it. To know if it’s cardiomyopathy, doctors look at the heart’s structure and function with imaging. If the heart muscle is enlarged, thickened, or stiff without other causes like blocked arteries or valve disease, it’s cardiomyopathy. That distinction guides treatment. You can have heart failure from a heart attack or high blood pressure-but those aren’t cardiomyopathies.
Final Thoughts
Cardiomyopathy isn’t a death sentence-but it’s not something to ignore. The three types are different diseases in disguise. One is stretched, one is thickened, one is stiff. Each needs its own map to treatment. The best outcomes come from knowing exactly what you’re dealing with-and acting fast.
With better testing, targeted drugs, and family screening, survival and quality of life have improved dramatically over the last decade. But progress only helps if people get diagnosed. If you or someone you love has unexplained heart symptoms, push for answers. Ask for the type. Ask for the cause. Ask for the plan. Because in cardiomyopathy, knowing the difference isn’t just medical-it’s life-saving.

Cinkoon Marketing
So I read this whole thing and honestly? I had no idea cardiomyopathy had three distinct flavors. I thought it was just 'weak heart.' Now I get why my uncle's treatment was so weird compared to my cousin's. DCM sounds like a deflated balloon, HCM like a muscle-bound gym bro, and RCM like a cardboard box trying to hold water. Wild.
Also, mavacamten? That's the drug that costs $145k? Jesus. We need better pricing. This isn't a luxury, it's a lifeline.