Every time you pick up a prescription for a generic drug, you’re holding a product that’s been engineered to be identical to its brand-name version - down to the last molecule. But how exactly do these cheaper pills get made? The process isn’t just about copying a formula. It’s a highly regulated, scientifically rigorous journey that takes years, millions of dollars, and precision at every step. And if any part of it fails, the entire batch gets thrown out.
Starting with the Brand-Name Drug
The journey of a generic drug begins with the brand-name version. Manufacturers don’t just guess what’s inside. They take the original product - called the Reference Listed Drug (RLD) - and break it apart. Using advanced lab techniques like HPLC (High-Performance Liquid Chromatography) and mass spectrometry, they identify the exact active ingredient, how much is in each tablet, and every inactive ingredient (excipients) like fillers, binders, and coatings. This reverse engineering is critical. Even small differences in these additives can change how the drug dissolves in your body.For example, if the brand-name drug uses a specific type of lactose with a certain particle size to control how fast the medicine releases, the generic maker must find an exact match. A 2024 Reddit thread from a pharmaceutical engineer with over a decade of experience highlighted this: “One supplier changed their lactose batch. Our tablets went from 12-minute dissolution to 28 minutes. We had to scrap 300,000 units.” That’s not a typo. A tiny change in an inactive ingredient can break the whole product.
Designing the Formula: Quality by Design
Once they know what the original drug is made of, manufacturers use a framework called Quality by Design (QbD), developed by the International Council for Harmonisation (ICH). This isn’t trial and error. It’s science-driven planning. They map out three key things:- Critical Quality Attributes (CQAs): What properties must the final drug have? Like how fast it dissolves, how stable it is over time, or how evenly the active ingredient is spread.
- Critical Material Attributes (CMAs): What properties of the raw ingredients affect those CQAs? Like the crystal shape of the active ingredient or the moisture content of the filler.
- Critical Process Parameters (CPPs): What steps in manufacturing must be tightly controlled? Like temperature during mixing, pressure during compression, or drying time.
Every single one of these is tested and documented. If the tablet’s dissolution rate is off by even 5%, the batch is rejected. The FDA requires that generic drugs release their active ingredient within the same time window as the brand-name version - usually 80% to 125% of the original’s rate.
The Manufacturing Steps: From Powder to Pill
The actual production of generic drugs follows a strict, seven-step sequence. Each step is monitored in real time, and every batch is tracked with digital records.- Formulation: The active pharmaceutical ingredient (API) is weighed precisely and mixed with excipients like starch, cellulose, or magnesium stearate. This isn’t just stirring. It’s done in controlled environments with ISO Class 5-8 cleanrooms to prevent contamination.
- Mixing and Granulation: The powder blend is turned into granules - small clumps that flow better and compress evenly. Wet granulation adds a liquid binder; dry granulation uses pressure alone. Both methods are validated to ensure consistency.
- Drying: If wet granulation was used, the granules are dried in ovens at exact temperatures (usually 40-60°C) for set durations. Too much heat? The active ingredient degrades. Too little? The tablets won’t hold together.
- Compression and Encapsulation: Dry granules are pressed into tablets using high-speed tablet presses. Each machine can make over 1 million tablets per hour. Capsules are filled using automated systems that drop precise amounts of powder into gelatin or vegetarian shells. Tablet weight must stay within ±5% for small tablets (under 130mg) or ±7.5% for larger ones, per FDA guidelines.
- Coating: Tablets get a thin coating to mask taste, protect from moisture, or control release. Some coatings are designed to dissolve only in the intestine, not the stomach. This layer must be uniform - no cracks, no bubbles, no uneven thickness.
- Quality Control: This isn’t a final check. It happens at every stage. Tablets are tested for hardness, thickness, dissolution rate, and active ingredient content. Random samples are sent to labs for purity checks. If one tablet fails, the whole batch is discarded.
- Packaging and Labeling: Bottles or blister packs are filled, sealed, and labeled. The label must match the brand-name drug’s exact wording - same warnings, same dosage instructions. But here’s the catch: the pill can’t look the same. U.S. trademark law forbids generics from copying the color, shape, or logo of the brand-name version. That’s why your generic Adderall might be white and oval, while the brand is orange and round.
Approval: The ANDA Pathway
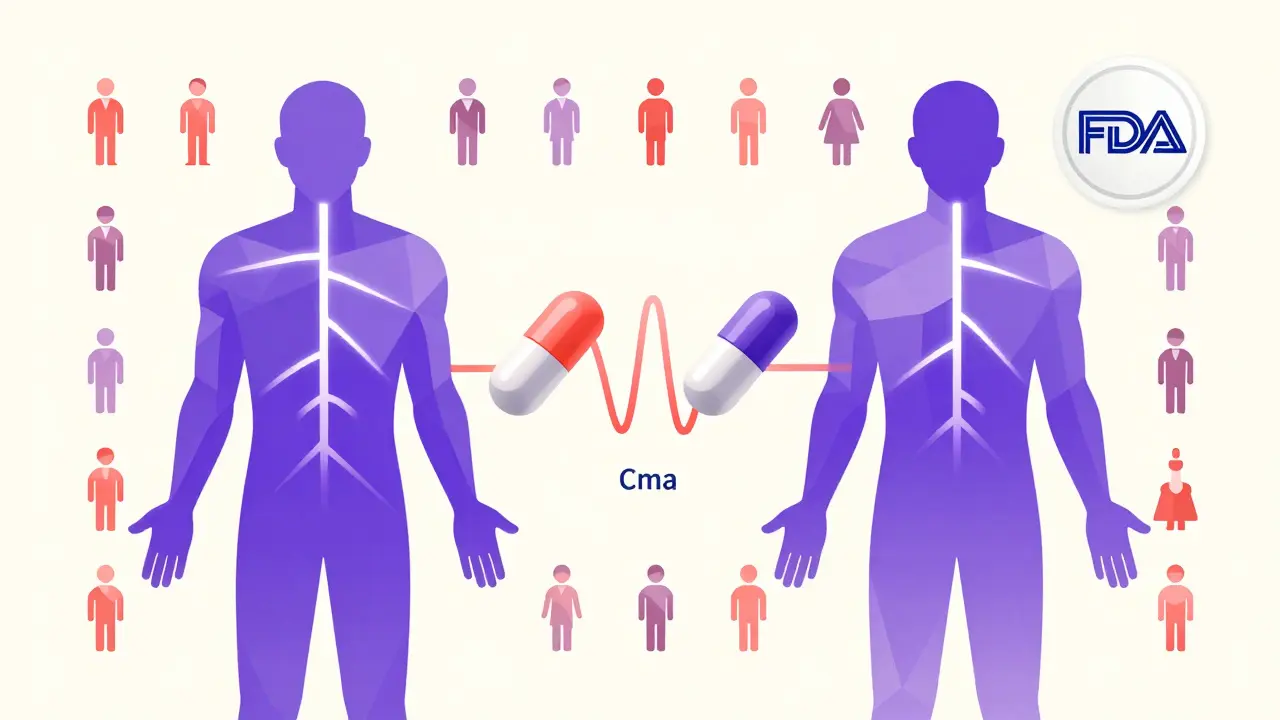
You can’t sell a generic drug without FDA approval. The shortcut? The Abbreviated New Drug Application (ANDA). Unlike brand-name drugs, which require 10-15 years and over $2 billion in clinical trials, generics skip human safety studies. Instead, they prove they’re bioequivalent.Bioequivalence means the generic drug enters your bloodstream at the same rate and amount as the brand-name version. This is tested in 24-36 healthy volunteers. Blood samples are taken over 24-72 hours to measure two key values:
- Cmax: The highest concentration of the drug in the blood.
- AUC: The total amount of drug absorbed over time.
The generic’s Cmax and AUC must fall within 80-125% of the brand’s values, with 90% confidence. That’s not a guess - it’s a statistical guarantee. If it doesn’t meet this, the FDA rejects the application.
The ANDA submission itself is massive - often 5,000 to 10,000 pages of data. It includes every analytical method used, every batch record, every stability test, and every bioequivalence report. The FDA reviews it in about 17 months on average, though complex drugs like inhalers or topical creams can take up to 3 years.
Manufacturing Standards: CGMP
Every facility making generic drugs must follow Current Good Manufacturing Practices (CGMP). These aren’t suggestions. They’re legal requirements. Facilities must maintain:- Temperature: 20-25°C
- Humidity: 45-65% relative humidity
- Cleanroom class: ISO 5 for sterile areas, ISO 7-8 for general production
Workers wear gowns, gloves, and masks. Air is filtered constantly. Equipment is cleaned and validated after every batch. Any deviation - even a temperature spike for 10 minutes - triggers a 24-hour investigation. If the root cause isn’t found and fixed, the FDA can shut the plant down.
In 2021, Teva had to recall 14 generic drugs after an FDA inspection found CGMP violations at its Puerto Rico plant. That’s not rare. According to FDA inspection data, 37% of warning letters to generic manufacturers cite failure to properly investigate out-of-specification results. That’s a red flag. It means someone saw a problem and didn’t fix the system that caused it.
Challenges and Real-World Issues
Not all generics are created equal - not because of the rules, but because of the complexity. Simple pills like metformin or lisinopril are easy to copy. But drugs like inhalers, topical creams, or extended-release capsules? Those are nightmares.A 2022 case study showed it took a company 7 years and $47 million to match the skin absorption of a generic version of Clobetasol Propionate, a steroid cream. Why? Because the original used a proprietary emulsion system. You can’t just swap ingredients - you have to rebuild the entire delivery mechanism.
And then there’s the supply chain. Nearly 80% of the active ingredients for U.S. generics come from China and India. A single natural disaster or trade policy change can cause shortages. In 2020, a factory fire in India led to a nationwide shortage of the antibiotic azithromycin.
Some experts, like Dr. Jerry Avorn from Harvard, worry about multi-source generics. If five companies make the same drug, each might use slightly different excipients or processes. For drugs with a narrow therapeutic index - like warfarin or lithium - even tiny differences in absorption can be dangerous. The FDA requires batch-to-batch consistency, but doesn’t require all generics to match each other - only the brand-name original.
Why It Matters
Generic drugs save the U.S. healthcare system over $1.7 trillion in the last decade. In 2023, 90% of all prescriptions filled were for generics. That’s not just convenience - it’s life-saving access. A cancer drug that costs $120,000 as a brand might drop to $30,000 as a generic. A hepatitis C cure that once cost $84,000 is now under $30,000 thanks to generics.And the system is getting faster. Under GDUFA IV (effective October 2022), the FDA now aims to review 90% of generic applications in under 10 months - down from 17. Continuous manufacturing, where drugs are made in a constant flow instead of in batches, is cutting production time from weeks to hours. Pfizer’s AI-powered visual inspection system cut inspection errors by 40% in trials.
But the real win? You don’t need to choose between affordability and safety. The FDA doesn’t lower its standards for generics. They’re held to the same exacting rules. The only difference is the price tag - and the billions of dollars saved every year because of it.
Are generic drugs as safe as brand-name drugs?
Yes. The FDA requires generic drugs to meet the same strict standards for quality, strength, purity, and stability as brand-name drugs. They must also prove bioequivalence - meaning they work the same way in your body. Over 90% of prescriptions in the U.S. are for generics, and studies show no meaningful difference in clinical outcomes for the vast majority of patients.
Why do generic pills look different from brand-name ones?
U.S. trademark laws prevent generic manufacturers from copying the color, shape, or logo of brand-name drugs. This avoids confusion and protects brand identity. But the active ingredient, dosage, and effectiveness are identical. The difference is purely cosmetic - like two brands of aspirin with different packaging.
Can generic drugs have different side effects?
The active ingredient causes the side effects - and that’s the same in generics. However, some people report differences due to inactive ingredients. For example, a filler like lactose might cause issues for those with severe intolerance. Or a coating might change how fast the drug releases, leading to temporary stomach upset. These cases are rare, but if you notice a change, talk to your pharmacist or doctor.
How long does it take to make a generic drug?
It typically takes 3-4 years and $5-10 million to develop and get approval for a simple generic drug. Complex generics - like inhalers or extended-release tablets - can take 7+ years and cost over $50 million. Most of that time is spent on testing, regulatory submission, and waiting for FDA review.
Are all generic drugs made in the U.S.?
No. About 80% of the active ingredients come from China and India. Final packaging often happens in the U.S. or Europe. The FDA inspects all facilities - foreign and domestic - before approving a drug. So location doesn’t determine quality. Compliance with CGMP standards does.
Why are some generic drugs more expensive than others?
Price depends on competition. If 10 companies make the same generic, prices drop fast - sometimes to pennies per pill. But if only one or two companies can make it - especially for complex drugs - prices stay higher. The FDA gives priority review to generics with no competitors, which helps drive down costs faster.
What happens if a generic drug fails inspection?
If the FDA finds serious violations - like poor sanitation, falsified records, or uncontrolled contamination - they issue a warning letter. The company must fix the issue or stop making the drug. In severe cases, the facility is banned from supplying the U.S. market. The FDA publishes inspection results publicly, so you can check if a manufacturer has a history of problems.
What’s Next for Generic Drugs?
The future of generics is moving toward complexity and innovation. More than 35% of pending applications are for hard-to-copy drugs like nasal sprays, injectables, and long-acting implants. The FDA is rolling out new guidance to help manufacturers navigate these challenges. AI, digital twins, and continuous manufacturing are becoming standard tools. And with $75 billion in branded drugs set to lose patent protection by 2027, the demand for generics will only grow.What hasn’t changed is the core promise: safe, effective medicine at a price people can afford. That’s not magic. It’s science, regulation, and relentless attention to detail - one pill at a time.


Erwin Kodiat
Man, I never realized how much science goes into those little white pills. I always thought generics were just cheap knockoffs. Turns out they’re basically the same engineering miracle as a SpaceX rocket-just smaller and with fewer explosions.