Switching from one digoxin generic to another might seem like a simple cost-saving move - but for patients with heart failure or atrial fibrillation, it can be dangerous. Digoxin isn’t like most medications. Even tiny changes in how much of the drug enters your bloodstream can mean the difference between effective treatment and life-threatening toxicity. This isn’t theory. It’s happening in clinics right now.
Why Digoxin Is Different
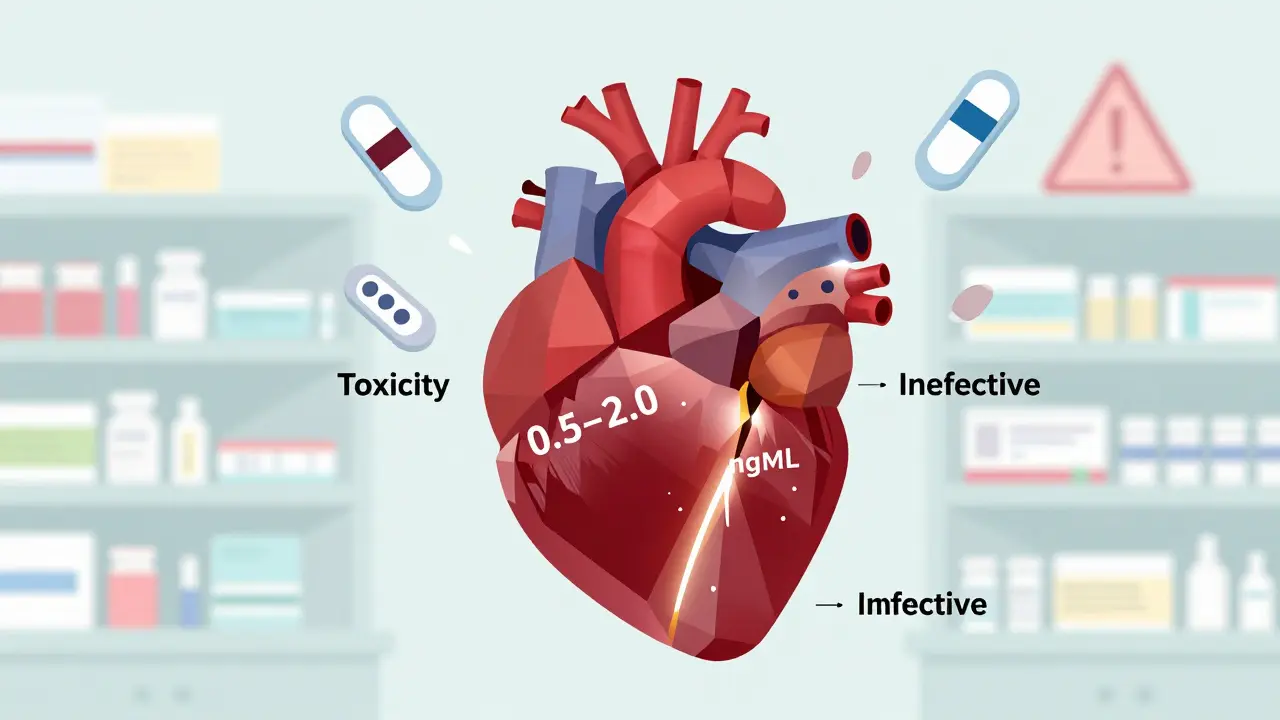
Digoxin is a cardiac glycoside. It helps slow down a racing heart and strengthens weak heart contractions. But it doesn’t take much to push it over the edge. The safe, effective range? Just 0.5 to 2.0 nanograms per milliliter of blood. Go below that, and the drug stops working. Go above it, and you risk nausea, vomiting, blurry yellow vision, irregular heartbeats, and even sudden cardiac arrest.
That’s what makes digoxin a narrow therapeutic index (NTI) drug - one of the most dangerous classes of medicines when it comes to dosing precision. Unlike antibiotics or blood pressure pills, where you have some wiggle room, digoxin leaves almost no room for error. And here’s the kicker: generic versions of digoxin aren’t all created equal, even if they’re labeled the same.
The FDA’s Special Rules for Digoxin
In 2002, the FDA took an unusual step. While most generics just need to prove they’re similar to the brand-name version, digoxin had to meet stricter standards. The agency required that any generic digoxin tablet must match the brand-name Lanoxin within a 90% confidence interval of 80-125% for two key measures: how much of the drug gets absorbed (AUC[0-12]) and how fast it reaches peak levels (Cmax).
That sounds strict - and it is. Most drugs only need to hit 80-125% on average. But for digoxin, the FDA demanded tighter control because of its history of inconsistent absorption. Some older generics had bioavailability as low as 45% in certain patients. That’s not just a small difference - it’s a clinical emergency waiting to happen.
Today, only three generic digoxin manufacturers in the U.S. carry an “AB” rating in the FDA’s Orange Book. That means they’ve passed the bioequivalence test. But here’s what no one tells you: those tests were done on healthy volunteers. Not elderly patients with kidney problems. Not people taking ten other medications. Not those with fluctuating diets or gut conditions that affect absorption.
The Real Problem: Switching Between Generics
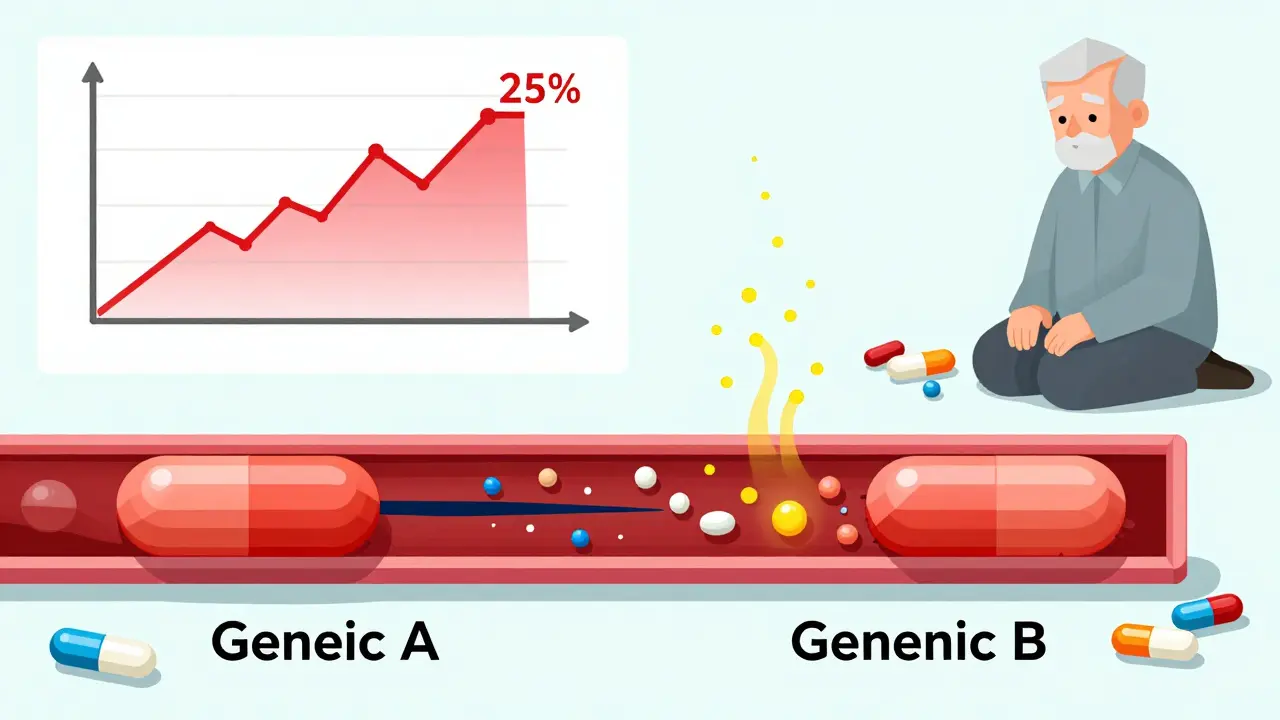
Here’s where things get risky. Let’s say you’ve been stable on Generic A for six months. Your doctor switches you to Generic B because your pharmacy changed suppliers. You feel fine. No symptoms. So you don’t get your blood tested.
But here’s what’s likely happening: Generic A and Generic B may both be “bioequivalent” to Lanoxin - but they haven’t been tested against each other. There’s no data proving they’re interchangeable. And studies show that switching between different generic manufacturers can cause serum digoxin levels to shift by more than 25% - enough to trigger toxicity or make the drug ineffective.
A 2023 review in US Pharmacist compared this to switching tacrolimus in transplant patients. No one does that without checking blood levels. Yet, doctors still switch digoxin generics all the time - often without even telling the patient.
Why? Because it’s cheaper. Pharmacy benefit managers push for the lowest-cost generic. But cost savings shouldn’t come at the risk of hospitalization or death.
Formulation Matters Too
Not all digoxin is made the same way. The tablet form has an average bioavailability of 60-80%. But the liquid form - digoxin elixir - absorbs much better, at 70-85% of the intravenous dose. That’s a big difference.
So if you’re switched from a tablet to an elixir - even if it’s the same manufacturer - your dose needs to be adjusted. The numbers don’t match up. A 0.125 mg tablet isn’t the same as 0.125 mg of liquid. Yet, many patients aren’t warned about this. They just get a new prescription and assume it’s identical.
Who’s Most at Risk?
Most digoxin users are older adults - often over 70. Many have reduced kidney function, which means the drug clears from their body slower. That increases the chance of buildup. Others are on multiple medications. Diuretics? They can lower potassium, which makes digoxin more toxic. Antibiotics? Some alter gut bacteria that affect absorption. Calcium channel blockers? They can raise digoxin levels.
And here’s the silent killer: inconsistent dosing. If you take your pill at different times each day, or skip meals, or have a stomach bug - your absorption changes. One day you get 70% of the dose. The next, only 40%. Over time, that leads to unpredictable levels.
What You Need to Do
If you’re on digoxin - whether brand or generic - here’s what you need to know:
- Stick to one manufacturer. Don’t let your pharmacy switch you without telling you. Ask: “Is this the same brand as before?”
- Get your blood tested. The American College of Clinical Pharmacy recommends checking serum levels 4-7 days after starting or changing digoxin. Repeat the test after any dose change, new medication, or change in kidney function.
- Know the signs of toxicity. Nausea, vomiting, loss of appetite, confusion, visual changes (especially yellow-green halos), or a slow or irregular heartbeat - call your doctor immediately.
- Don’t assume generics are the same. Even if they look identical, different manufacturers use different fillers, binders, and coatings. These affect how the pill dissolves in your gut.
- Ask about the elixir. If you have trouble swallowing pills, ask if liquid digoxin is right for you - but only if your doctor adjusts the dose properly.

What Doctors Should Be Doing
Guidelines from the American Heart Association and American College of Cardiology are clear: Use the same manufacturer’s product consistently. If you must switch, monitor serum levels 3-5 days after the change.
Yet, many clinicians still don’t check digoxin levels routinely. They rely on clinical symptoms alone. That’s not enough. By the time a patient feels sick, the level may already be dangerously high.
Best practice? Check a trough level - drawn just before the next dose - at least once after initiation, and again after any change. Keep the result on file. If the level is below 0.5 ng/mL, the drug may not be working. If it’s above 1.2 ng/mL, especially in heart failure patients, mortality risk goes up.
Recent evidence suggests the ideal target range for heart failure is now 0.5-0.9 ng/mL. Higher levels don’t improve outcomes - they just increase risk.
What’s Being Done?
The FDA still treats digoxin as a high-risk generic. Manufacturers must submit detailed dissolution profiles for each batch. They’re required to test for consistency across batches - not just against the brand, but within their own production lines.
Some countries, like Estonia, have successfully integrated generic digoxin into routine care after confirming bioequivalence. But they also enforce strict monitoring protocols. In the U.S., that’s not standard.
Until every pharmacy, doctor, and patient understands that digoxin isn’t just another pill - we’ll keep seeing preventable hospitalizations.
Bottom Line
Digoxin generics are not interchangeable. Even if they’re labeled the same, they’re not the same. The science says they can be bioequivalent to the brand - but not to each other. And for a drug with such a narrow safety window, that’s enough to kill someone.
Don’t let cost savings override safety. Ask your pharmacist: “Is this the same brand I’ve been taking?” If they say yes, ask for the name of the manufacturer. Write it down. Stick with it. And if you’re switched - demand a blood test.
Your heart doesn’t care about insurance formularies. It only cares about the exact amount of digoxin in your blood - and whether it’s in the safe zone.
Are all generic digoxin tablets the same?
No. While each generic must meet FDA bioequivalence standards compared to the brand-name Lanoxin, they haven’t been tested against each other. Switching between different generic manufacturers can cause significant changes in blood levels - even if both are labeled "digoxin 0.125 mg."
How often should digoxin levels be checked?
Check serum digoxin levels 4-7 days after starting therapy or changing the dose. After switching between different generic products, test again 3-5 days later. Also test whenever kidney function changes, new medications are added, or if symptoms of toxicity or poor control appear.
Can I switch from a digoxin tablet to the liquid form?
Yes - but only if your doctor adjusts the dose. The elixir is more bioavailable (70-85%) than tablets (60-80%). A 0.125 mg tablet is not equal to 0.125 mg of liquid. Never swap forms without a dose recalibration.
What are the signs of digoxin toxicity?
Common signs include nausea, vomiting, loss of appetite, confusion, visual disturbances (yellow or green halos around lights), and irregular heartbeat - especially bradycardia or new arrhythmias. These symptoms can appear even if blood levels are only slightly above normal.
Why is digoxin riskier for older adults?
Older adults often have reduced kidney function, which slows digoxin clearance. They’re also more likely to take other medications that interact with digoxin - like diuretics, antibiotics, or heart rhythm drugs. This combination increases the risk of toxicity even at "normal" blood levels.
Is there a safer alternative to digoxin?
For heart failure, newer drugs like sacubitril/valsartan or SGLT2 inhibitors are now preferred as first-line treatments. For atrial fibrillation, rate control can often be managed with beta-blockers or calcium channel blockers. Digoxin is now typically reserved for patients who don’t respond to these options - or who need symptom relief despite optimal therapy.
Can I trust pharmacy substitutions for digoxin?
No. Automatic substitutions are risky. Always ask your pharmacist if the dispensed product is the same brand and manufacturer as your previous prescription. If it’s not, request the original or ask your doctor to write "Dispense as written" on the prescription.

Erica Vest
Digoxin is one of those drugs that should come with a warning label you can’t ignore. I’ve seen patients crash into toxicity because a pharmacy swapped generics without a word. The FDA’s AB rating doesn’t mean interchangeable-it means it’s equivalent to the brand, not to other generics. If your doctor doesn’t check levels after a switch, they’re not doing their job. This isn’t just theory. I’ve had two patients in the ER last year because of this exact issue.